Articles tagged with: Revlimid
News»

Elderly people with multiple myeloma have better treatment options now than ten years ago. In the past decade, the introduction of thalidomide (Thalomid), Velcade (bortezomib), and Revlimid (lenalidomide) have improved patients’ response to treatment and increased survival time, even when used without stem cell transplants.
In the July issue of Current Opinion in Hematology, Dr. Donna E. Reece, a physician and researcher at the Princess Margaret Hospital in Toronto, reviewed the current approaches to treating newly diagnosed multiple myeloma patients. Dr. Reece discussed autologous and allogeneic stem cell transplants …
News»
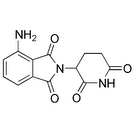
At the upcoming meeting of the American Society of Hematology (ASH), there will be several presentations about Actimid (pomalidomide). Actimid, a new drug being developed by Celgene Corporation as a treatment for multiple myeloma, is a molecular derivative of thalidomide (Thalomid).
Actimid As A Combination Therapy With Dexamethasone
One of the ASH presentations will show that the combination regimen of Actimid and low-dose dexamethasone (Decadron) is an effective treatment for relapsed multiple myeloma.
Actimid was tested in combination with dexamethasone in 34 patients with relapsed myeloma following therapy with
NewsFlash »
Innate Pharma And Celgene To Collaborate On Trial Combining Revlimid With IPH 2101 Therapies – Innate Pharma and Celgene will collaborate to evaluate the clinical potential of Celgene’s Revlimid (lenalidomide) plus Innate Pharma’s IPH 2101 in myeloma patients who have failed first-line therapy. In vitro studies on myeloma cell lines have shown that the two drugs may have a synergistic effect. Innate Pharma hopes to submit a request with the Food and Drug Administration in early 2010 to receive authorization to administer the drugs in humans. For more information, please see the Innate Pharma press release.
Myeloma UK Launches The Innovative Myeloma Clinical Trial Network – The Clinical Trial Network will design and manage a portfolio of early phase trials of myeloma drugs by drawing on a collaboration of clinical specialists, researchers, pharmaceutical companies, and the National Health Service’s regulatory bodies. Eight research centers around the UK will be involved in the trials included in the portfolio. Myeloma patients will be able to take part in the trials at these centers. The first trials are expected to begin recruiting patients in early 2010. For more information, please visit the Myeloma UK Web site.
Peter Boyle Memorial Raises $700,000 For The IMF – The Third Annual Peter Boyle Memorial raised $700,000 for the International Myeloma Foundation. The event was launched three years ago by Boyle’s widow to help fund the battle against multiple myeloma. For more information, please visit the Los Angeles Times Web site.
NewsFlash »
Dr. Ken Anderson Elected Officer To American Society Of Hematology – Dr. Ken Anderson is one of five newly elected officers to the American Society of Hematology’s (ASH) Executive Committee. Dr. Anderson is a renowned hematologist specializing in the treatment of multiple myeloma. He will begin serving his four-year term in January 2010. For more information, please see the ASH press release.
Revlimid Covered By Australian Government – Revlimid (lenalidomide) is now listed for the treatment of myeloma on the Pharmaceutical Benefits Scheme, a list of drugs subsidized by the Australian government. Revlimid will be subsidized for the next four years at a cost of A$104 million (US$94 million). For more information, please visit the Investor Village Web site.
Potential New Myeloma Treatment Starts Phase 1 Trial Extension For AML – Innate Pharma received approval from the French Regulatory authorities to begin an extension of the Phase 1 trial with IPH 2101 in patients with acute myeloid leukemia (AML). The company recently also started Phase 2 clinical trials with IPH 2101 for multiple myeloma. For more information, please see the Innate Pharma press release (pdf).
MMRF Race For Research – On November 15, the Multiple Myeloma Research Foundation (MMRF) is sponsoring a 5K walk/run. It will be held in Alexandria, VA and will start at 7:30 a.m. The MMRF is looking for participants and sponsors. For more information, please visit the MMRF Web site.
For a more detailed listing of myeloma related events, please check the Myeloma Beacon Events Calendar.
News»

A recent study of newly diagnosed multiple myeloma patients found that Revlimid (lenalidomide) and low-dose dexamethasone (Decadron) resulted in better short-term overall survival and fewer severe side effects than Revlimid and high-dose dexamethasone. Results were so convincing after one year that the trial was stopped early.
For years, Revlimid plus high-dose dexamethasone has been a standard treatment for multiple myeloma.
“The results of the study have already changed practice worldwide,” said Dr. S. Vincent Rajkumar of the Mayo Clinic and lead author of the study.
In this study, 445 patients …
News»

The International Myeloma Working Group (IMWG) recommends that multiple myeloma patients should have their stem cells collected within four cycles of therapy with Revlimid (lenalidomide), thalidomide (Thalomid), or Velcade (bortezomib). Otherwise, these drugs may interfere with stem cell collection. The recommendations were published in the journal Blood in August.
During an autologous stem cell transplant, physicians collect a patient’s stem cells in the early stages of the patient's anti-myeloma drug regimen, and return these same cells to the individual after the regimen is complete. Transplantation is a common treatment usually given to …
News»

Patients with multiple myeloma and other blood cancers have a high risk of venous thromboembolism, or blood clotting, according to a recent study published in the Journal of Clinical Oncology. The rate of this complication is influenced by multiple factors, including the type of disease, the type of chemotherapy, and the use of catheters.
Venous thromboembolism (VTE) is a condition in which a blood clot forms in a vein, limiting the blood flow in the affected vein. Additionally, a piece of the clot can break off, travel through the vein, and block …

