Researchers have published new results showing that patients with relapsed or refractory multiple myeloma achieve a better response to treatment with Revlimid (lenalidomide) plus dexamethasone than to dexamethasone alone. This finding holds for all patients, including those who have previously been treated with thalidomide (marketed as Thalomid).
The new results are based on pooled data from two randomized clinical trials involving more than seven hundred myeloma patients. The patients had either relapsed multiple myeloma - disease …
Read the full story »
Postgraduate Institute For Medicine Sponsors Conference On New Multiple Myeloma Treatments - In conjunction with the International Myeloma Foundation (IMF), the Postgraduate Institute for Medicine is sponsoring a December 5 symposium entitled, "Multiple Myeloma: Finding Your Way Through the Treatment Maze--Selecting the Best Treatment in the Era of Novel Agents." Hosted from 6:00 - 9:00 p.m. in San Francisco's Moscone Center, the program will provide an overview of the most recent clinical trials' findings, including results on the newest therapeutic …
Read the full story »
CIGNA Pharmacy Management (CIGNA) has joined forces with The Leukemia & Lymphoma Society (LLS) to promote the LLS financial aid program for patients with multiple myeloma and other blood cancers.
The LLS program assists blood cancer patients and their families by helping cover the costs of private health insurance premiums, co-payment obligations, Medicare Part B and Part D, Medicare Supplementary Health Insurance, and Medicare Advantage premiums and co-payment obligations. The aid package provides up to $5,000 …
Read the full story »
The FDA last Friday granted approval to Cephalon, Inc. to market the drug Treanda (bendamustine) for the treatment of indolent non-Hodgkin’s lymphoma (NHL).
Just seven months ago, the FDA also approved Treanda for the treatment of chronic lymphocytic leukemia (CLL), making Treanda the only new drug this year to gain FDA approval for two cancer indications.
Treanda is not officially approved in the United States for the treatment of multiple myeloma. It is, however, approved …
Read the full story »
 Thalidomide is a sedative-hypnotic, and multiple myeloma medication. The drug is a potent teratogen in rats, rabbits, and primates including humans: this means that severe birth defects may result if the drug is taken during pregnancy. [...]
Thalidomide is a sedative-hypnotic, and multiple myeloma medication. The drug is a potent teratogen in rats, rabbits, and primates including humans: this means that severe birth defects may result if the drug is taken during pregnancy. [...]Read the full story »
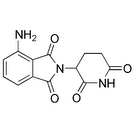
| Brand Name: | |
| Generic Name: | pomalidomide |
| Code Name: | CC-4047, Actimid |
| Company: | Celgene |
| FDA Clinical Phase: | 1/2 |
Description:
Pomalidomide (new articles, forum discussions) is an immunomodulatory agent (a drug that affects the immune system) that encourages a patient’s immune system to attack and destroy myeloma cells.Read the full story »

